Log in
Log into your PedsMrkt account to access our Community forums.
If you don't see an email, please check your Junk folder. The email will be sent from support@pedsmrkt.com
Log into your PedsMrkt account to access our Community forums.
If you don't see an email, please check your Junk folder. The email will be sent from support@pedsmrkt.com
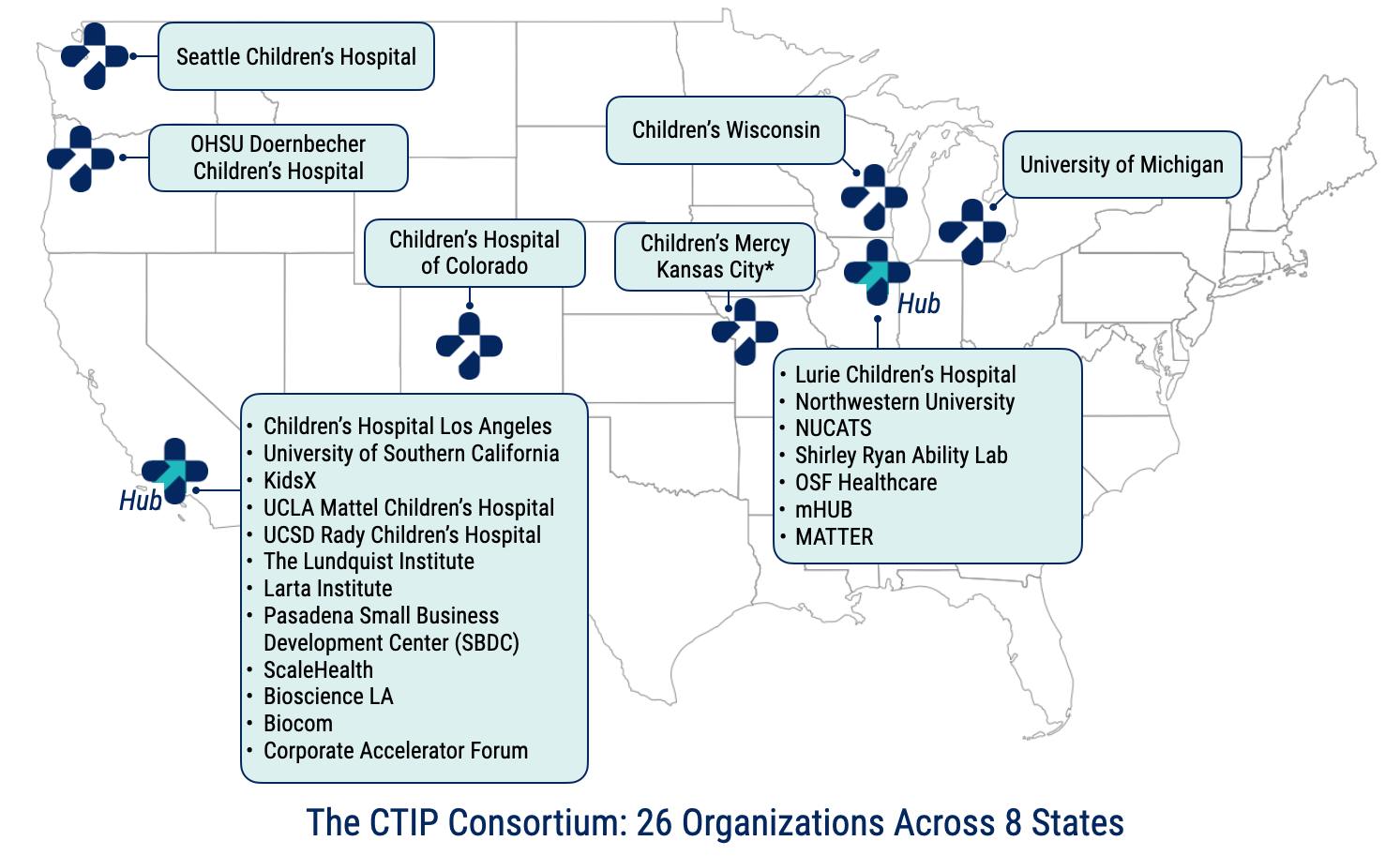
The Consortium for Technology & Innovation in Pediatrics (CTIP) is a pediatric medical device accelerator based at Lurie Children’s Hospital (LCH) and Children's Hospital Los Angeles (CHLA). Established in 2011, CTIP has been funded by the U.S. Food and Drug Administration Pediatric Device Consortia (PDC) grant program in 2013, 2018, and 2023.
CTIP promotes the development and commercialization of pediatric medical devices by simultaneously engaging and coordinating clinicians, engineers, regulators, hospital administrators, industry, patients, and the business community to guide and support medical device development for children.
CTIP has established a robust network of children’s hospitals, academic institutions, accelerators, incubators, and ecosystem partners to support the commercialization of pediatric medical devices. Over the past ten years, CTIP’s Network has steadily grown from local to national membership, with 26 institutions across 8 states participating in the 2023-2028 cycle.
CTIP aims to sustain a needs-driven pipeline of new pediatric medical devices and to develop successful models to increase the likelihood of commercializing these medical devices for children.
CTIP provides feasibility analysis tools, resources, and services as well as assembles and educates pediatric product development teams. CTIP is dedicated to promoting and advancing the commercialization of pediatric medical devices by serving innovators and companies and by engaging clinicians, engineers, patients and the business community in the process of assessment and development of novel technologies related to pediatric care. CTIP continually seeks to build its relationships with a variety of stakeholders, such as the medical device industry, medical centers, innovators, parents, and patient advocacy groups. CTIP has supported over 200 pediatric device projects since its inception. Today CTIP’s active portfolio includes over 100 technologies and pediatric medical devices.
Boppli®: Accurate, non-invasive, low risk, convenient blood pressure monitoring for infants Bopp...
Over 90% of patients get an IV, and more than half of those fail before their useful life due to ...
Remmie 4 is the only FDA-registered USB-smart otoscope for easy, at-home ear exams on phones, tab...
Developed by a neonatologist, the smallTalk NICU Egg is a thoughtfully designed speaker tailored ...